How Does Temperature Affect the Keq of a Reaction
You seem to be saying that the only way you can change P is to change Keq. This will increase the concentrations of the products and decrease the concentrations of the reactants.
Why Is Equilibrium Constant Affected By The Temperature But Not Affected By The Concentration Of Reactants Quora
If you increase temp for endothermic rxn forward direction the Keq will increase because product is favoured.

. If you increase the temp the system will not favor the products. The yield of ammonia NH3 NH 3 will decrease. When KEQ is 1 What is Delta G.
An investigation of all the factors that can affect the equilibrium constant of a chemical reaction. The Effect of Temperature on Keq. Top Madeline Louie 1B Posts.
Changing the temperature favours one reaction over the other. In this post we qualitatively analyse the effect of temperature on the value of Keq. It only changes for temperature.
It is a product of the reaction. For gas phase reactions usually concentration is measured not in moles per liter but as pressure invoking Daltons Law of partial pressures. Decreasing the temperature causes the Keq to increase.
If you increase temp for exothermic rxn forward direction the Keq will decrease because the reactant is favoured. For a reaction involving a single reactant and single product the equilibrium concentrations of reactant and product are unaffected by their initial concentrations. Here is the Arrhenius Equation on the temperature dependence of the rate of a chemical reaction.
As T increases the value of the exponential part of the equation becomes less negative thus increasing the value of k. Thus k2 k1. Keq HCl2 H2 Cl2 As the equilibrium shifts to the left the HCl goes down and both the H2 and Cl2 increase.
For an endothermic reaction increasing the temperature will always shift the reaction to the right or toward the products. Up to 24 cash back Keq is the same for a given reaction that is. Change of temperature of the system.
Increasing the temperature increases the rate constant If the temperature is lowered then T2 T1 and the right side of the equation is negative. The Keq is a mathematical constant that does not change for concentration volume and pressure changes. At constant temperature changing the equilibrium concentration does not affect Keq because the rate constants are not affected by the concentration changes.
At constant temperature changing the equilibrium concentration does not affect Keq because the rate constants are not affected by the concentration changes. Increasing temperature by heating up a reaction mixture of reactants and products at equilibrium will also increase the value of Keq ie. N2g 3H2g 2NH3g ΔH 92 N 2 g 3H 2 g 2NH 3 g Δ H 92 kJ kJ An increase in temperature.
Factors that influence the value of Kc K c Concentration pressure and temperature all affect the equilibrium position of a reaction and a catalyst affects reaction rates. Changing the temperature favours one reaction over the other. Lets say that before changing the temperature QK001 just an example.
An investigation and explanation of equilibrium disturbances on the. At equilibrium at the same temperature no matter what the initial concentrations were. Just realize however the rate constant is altered by temperature frequency of collision and number of collisions with proper orientation.
If you decrease the temperature it would be more favorable that the rxn proceeds to the right the equilibrium itself shifts to the right and therefore the Keq will be increased. Because a catalyst accelerates the forward and reverse reactions to the same degree it does not change the Keq of a reaction. A system in equilibrium is affected by the following factors.
The effect of temperature on Keq At two different temperatures. The temperature will affect the value of K. More products formed if.
Why does concentration not affect KC. Where k rate constant of the reaction A Arrhenius Constant Ea Activation Energy for the reaction in Joules mol 1 R Universal Gas Constant T Temperature in. All of these influence the rate constant.
If the temperature is increased in an endothermic reaction K will increase but in an exothermic reaction K will decrease. Increasing the temperature of a reaction generally speeds up the process increases the rate because the rate constant increases according to the Arrhenius Equation. Answer 1 of 2.
An exothermic rxn will release heat to the environment when the reaction proceeds. Addition of some inert gas. At constant temperature changing the equilibrium concentration does not affect Keq because the rate constants are not affected by the concentration changes.
When you increase the temperature what happens is that the equilibrium constant K drops. The reverse reaction is endothermic so the reverse reaction is favoured. What affects KEQ.
Delta G. The Effect of Temperature on Keq. As long as the pressures are fixed the temperature does not affect the instantaneous value of the reaction quotient.
Answer make it smaller. So your Keq would be in units of pressure units-1 say Pa-1. The Keqdecreases in value and heat is added to an exothermic reaction.
N2O4g heat 2NO2g. Favours the endothermic reaction because it takes in energy cools the container. A B C energy Increasing the temperature causes the Keq to decrease.
Ln k2k1 -EaR1T2 1T1 If the temperature is raised then T2 T1 and the right side of the equation is positive. Mainly on how temperature can affect the exothermic and endothermic nature of chemical equilibriums. Instead they reach a state of equilibrium at a given temperature where the rate of the forward reaction equals the rate of the reverse reaction.
Lets say Keq a²bc² and abc P. Its value is defined by Arrehenious equation an equation you dont necessarily need to know. Keq changes with temperature.
The Effect of Temperature on Keq. Change of concentration of any reactant or product. This is because by raising the temperature in an endothermic reaction you are favoring the creation of the products for that reaction.
Changing the temperature favours one reaction over the other. This point of equilibrium is known as the Keq value and can be defined as the ratio of the concentration of products to the concentration of reactants at equilibrium. The actual rate of the reaction varies with concentration of reactant.
Change of pressure of the system. However only temperature temperature affects the value of Kc K c. This makes the numerator smaller and the denominator larger.

4 The Equilibrium Constant Keq Labxchange
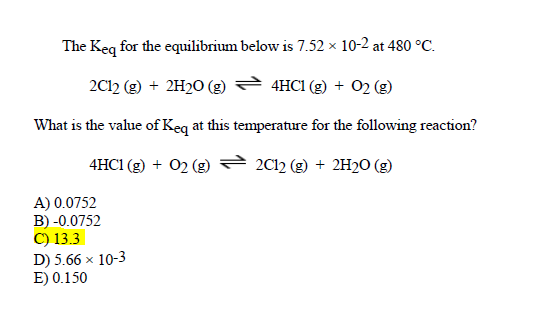
Solved The Keq For The Equilibrium Below Is 7 52 X 10 2 At Chegg Com

Unit 4 Chemical Equilibrium K Kc Or Keq Ppt Download
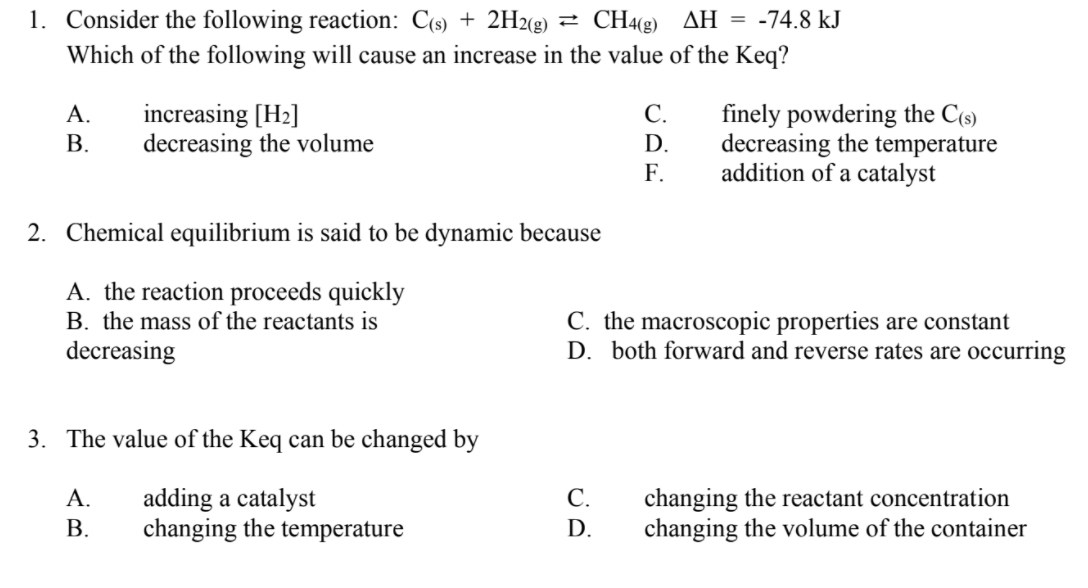
Solved 1 Consider The Following Reaction C S 2h2 G Chegg Com
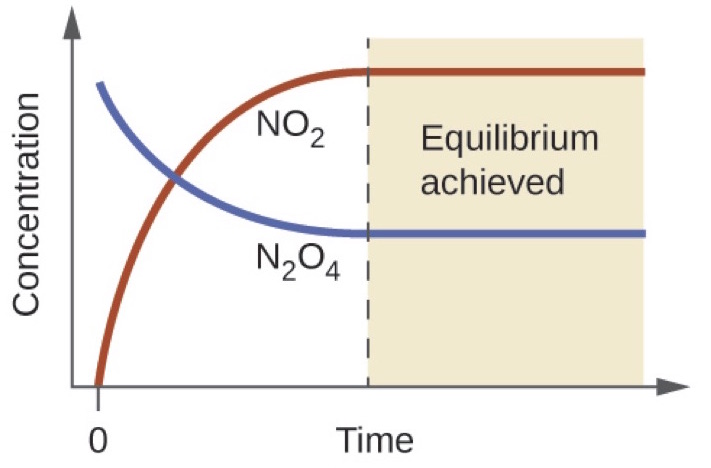
The Equilibrium Constant K Article Khan Academy

Effect Of Temperature On Equilibrium

Equilibrium Ii 15 6 Using The Equilibrium Constant Keq Calculating
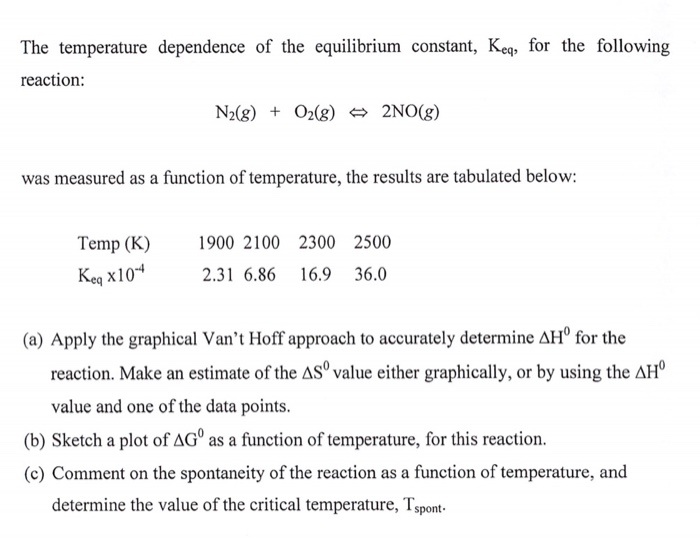
Solved The Temperature Dependence Of The Equilibrium Chegg Com

Log Of The Equilibrium Constant As A Function Of 1 Temperature Keq T Download Scientific Diagram
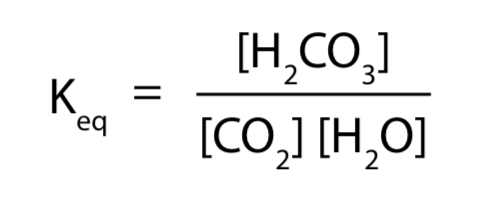
4 The Equilibrium Constant Keq Labxchange

Temperature Dependence Of Equilibrium Constant Youtube
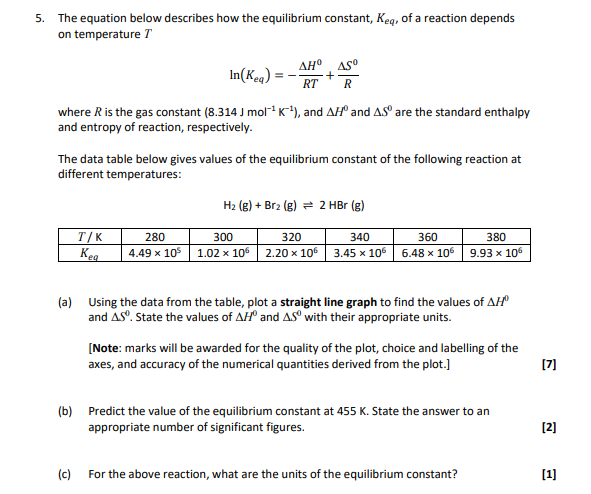
Solved 5 The Equation Below Describes How The Equilibrium Chegg Com

Thermodynamics Effect Of Temperature On Equilibrium Constant In Terms Of Entropy Change Chemistry Stack Exchange
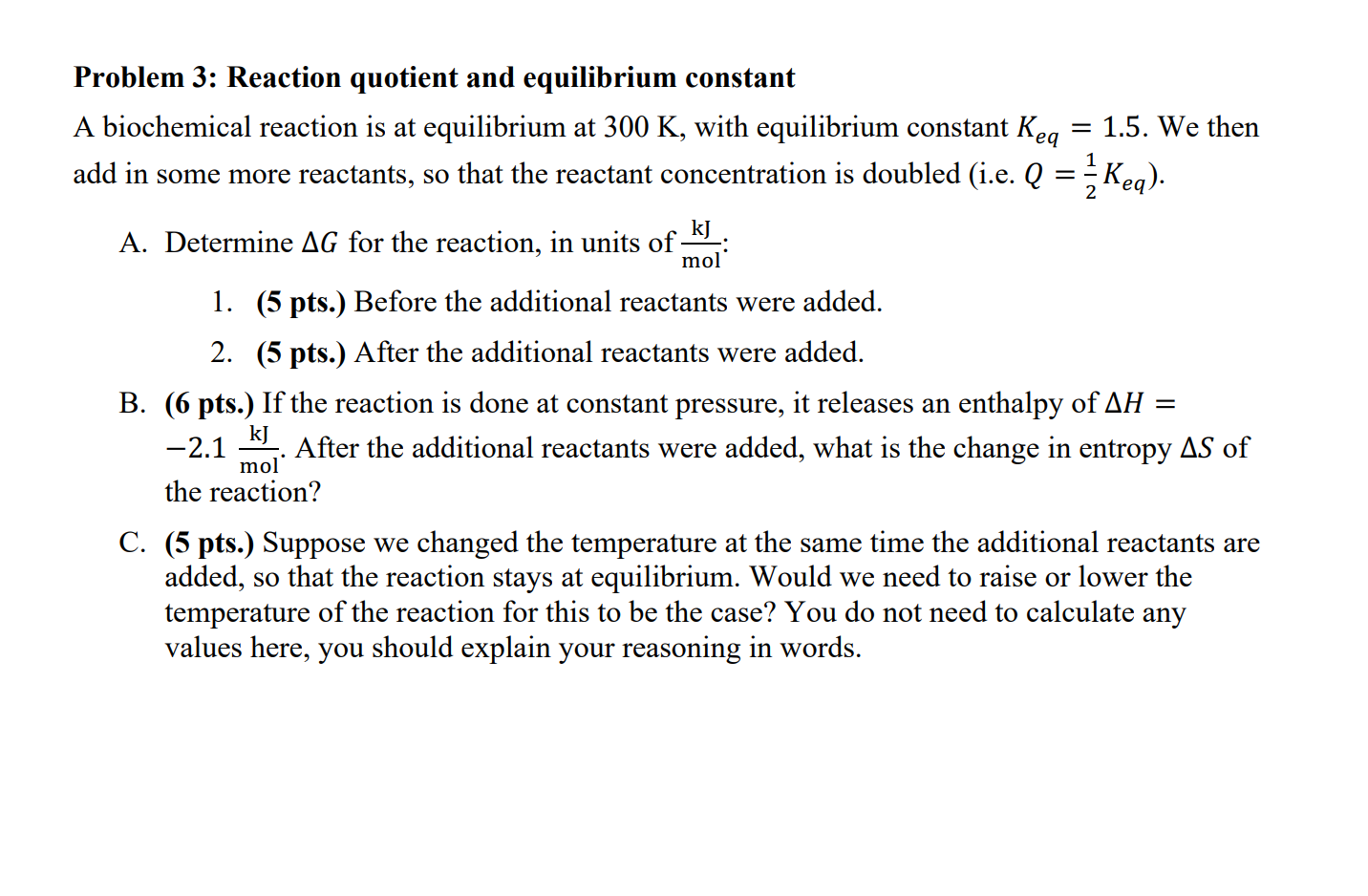
Solved Problem 3 Reaction Quotient And Equilibrium Constant Chegg Com

Solved E Calculate Keq At Standard Conditions Does The Forward Reaction Favor Reactants Or Products Explain Your Answer F Calculate The Pressure Of Coz G At Standard Conditions G Should Temperature Increase Or Decrease

Chemistry Life The Universe And Everything
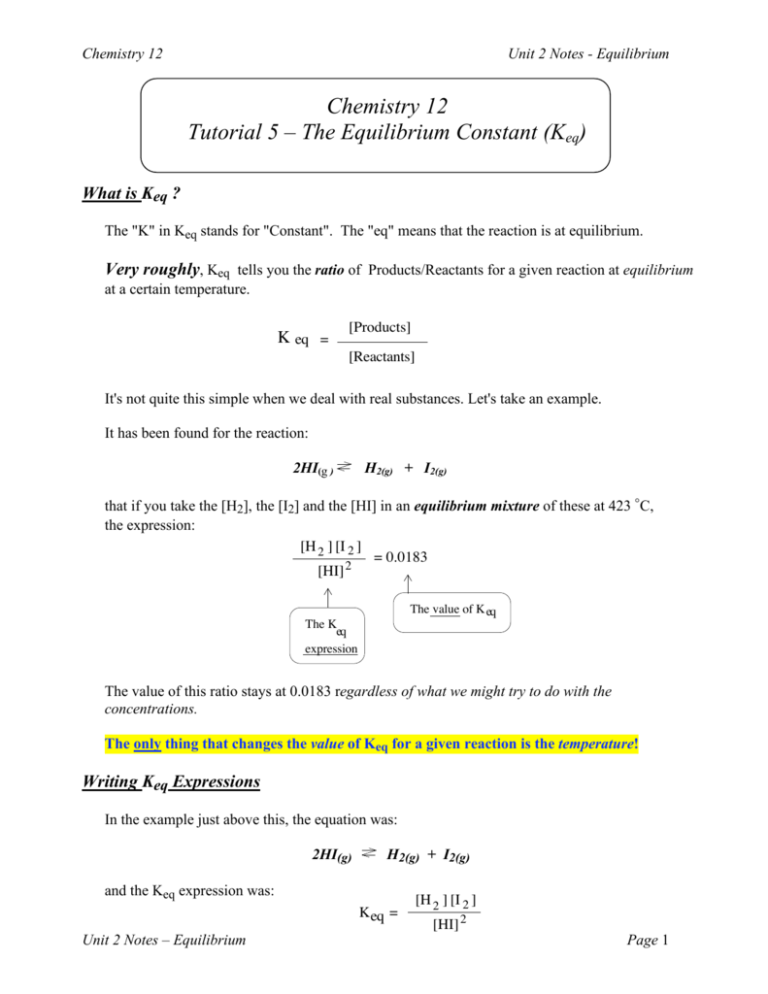
Chemistry 12 Tutorial 5 The Equilibrium Constant Keq

Reaction Quotient Q And Effect Of Temperature On Keq Hsc Chemistry Youtube

Comments
Post a Comment